The chemical reactions of organic compounds are a key part of this unit and its assessment. There are a few different types of reactions. We need to be able to classify reactions as being one (or more) of these:

When water (usually in the presence of an acid of alkali) breaks apart a molecule or polymer, we call this hydrolysis.
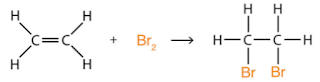
Addition Reactions
These occur when a double (or triple) bond breaks and a molecule (such as HCl) is added:
Hydrogenation is a special example, where hydrogen is added. It is also an example of reduction:
Hydration is another special example of an addition reaction:
Addition Polymerisation is also an example of this.
Addition reactions sometimes require us to consider Markovnikov's Rule:
Elimination Reactions
These occur when something is removed from a compound (or pair of compounds). It usually creates a double (or triple) bond.
We often have to consider Saytzeff's Rule when elimination occurs:
Condensation Polymerisation is also an example of this.
Substitution Reactions
As the name suggests, something has to be removed from the compounds before something new can be added to it. These are most common when the carbon atoms are already saturated (only have single C-C bonds). Sometimes quite a lot of energy is required to break the bond(s) to remove part of the compound.
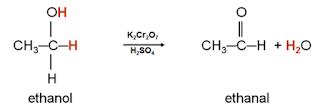
Oxidation-Reduction Reactions
Technically, redox is the transfer of electrons (oxidation = loss of electrons; reduction = gain of electrons). However in Organic Chemistry, our definition of "redox" is quite simple:
Oxidation = addition of oxygen and/or loss of hydrogen
Reduction = loss of oxygen and/or gain of hydrogen
Polymerisation Reactions
Polymerisation is when many ("poly-) smaller units ("-mer") combine to make a macromolecule, called a polymer. The smaller units are called monomers. There are two types of polymerisation:
ADDITION POLYMERISATION
When monomers react by breaking a double (or triple bond), also called an unsaturated carbon-carbon bond.
When monomers react by breaking a double (or triple bond), also called an unsaturated carbon-carbon bond.
CONDENSATION POLYMERISATION
The removal of a small molecule (usually water) to create a polymer. It can be from one monomer or two different monomers (as show):








Comments
Post a Comment