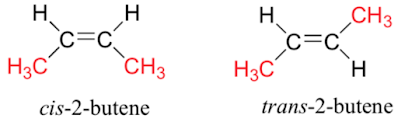
Alkenes can have different arrangements of atoms for the same molecular formula, due to the inability of the double bond to rotate. This is best shown in 2-butene:
The largest carbon chain either goes on the same side of the double bond (cis) or across the double bond (trans).We are often asked to explain why a compound does (or does not) exhibit geometric isomerism. We say that each carbon in the double bond has two different groups on it. If one of the carbon atoms has the same group bound to it, it will not exhibit geometric isomerism:

Comments
Post a Comment