Alkanes are the simplest organic compounds, made only of carbon and hydrogen atoms, and only containing single C-C bonds. The name we use for compounds containing only hydrogen and carbon is hydrocarbon.
NOMENCLATURE (Naming)
We name organic compounds based upon the longest carbon chain. There are prefixes used for these:
 |
| SOURCE: https://www.acegamsat.com/gamsat-organic-chemistry/ |
ISOMERISM
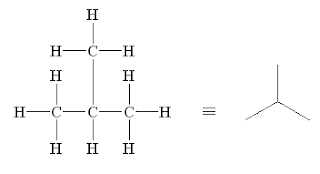
Alkanes often exhibit structural isomerism. This is where two (or more) compounds have the same formula (carbon and hydrogen atoms in this case) but different arrangements of those atoms:
To name these, you first find (and name) the longest carbon chain. You then number the atoms to assign where the side chains (branching) is.
CONDENSED FORMULAE
We often condense the formula of alkanes. This is because we know the only atoms are carbon and hydrogen, and all of the bonds are either C-H or C-C (single bonds).
Condensed Structural Formulae
These group sections of the structure that are the same. For example:
CH3-CH2-CH2-CH2-CH3 is often written as CH3(CH2)3CH3
Skeletal Structure
These structures are even more useful. Each line shows a C-C bond and the hydrogen atoms are removed. These show branching very well, and can help us link the structure to NMR peaks and Mass Spec fragments.




Comments
Post a Comment